Listen to the bonus interview:
Katie Luu: MIT, this is the Energy Initiative. I’m Katie Luu. Today we’re talking with Karthish Manthiram, professor of chemical engineering at MIT.
You just released a paper about a new and greener way to make a specific raw material. I had never heard of this material before but it seems to be in everything, from clothing and detergents to pharmaceuticals and plastics. Can you tell us what it is and what’s in it?
Karthish Manthiram: We’ve developed a new route for making epoxides. These are chemicals that probably most of us haven’t heard of but that are involved in virtually everything that we touch in a given day. Whether it’s our clothing, a plastic bottle that we’re drinking water out of, these are places in which epoxides feature prominently. It’s hard to imagine our everyday lives going on without them, yet these epoxides have an enormous CO2 footprint built into their production. That’s something that we set out to solve, is to figure out a way of getting rid of those CO2 emissions so that we could have greener chemicals and materials involved in the production of things that we use in our everyday lives.
KL: How would you say epoxides rank in terms of other CO2-producing processes?
KM: Epoxides have the fifth largest CO2 footprint of any commodity chemical that’s made today. If we want to have a big impact in terms of getting rid of these embedded CO2 emissions, it’s one of the good starting points.
KL: Besides the obvious climate change connection, how does this problem affect the average person?
KM: I think that the CO2 emissions are one important part of the problem but there are a few other aspects that we can consider as well. One example, for instance, is the safety involved in making a given chemical. A lot of these chemicals are made at elevated temperatures, elevated pressures, or they involve intermediates that can be relatively difficult to handle. Things like peroxides, which often create explosions. In addition to the CO2 footprint, there’s a safety aspect tied in there. One can also look, for instance, at the ability to make something on demand at the point of need. You can imagine going online and ordering a product and it shows up a few days later, or you could fire up a 3D printer and make it right there.
The same thing is also true for the chemicals and materials that we need. We can either wait for them to get from someplace in which they’re produced to where we need them, often going through some kind of inventory and transit and transportation steps, or we could make them right there when we want them. That’s an added benefit of many of the routes that we’re developing. CO2 emissions, safety, and the ability to make things on demand are all reasons for which we want to try and pursue the kinds of methods that we’re developing.
KL: What are the problems with how epoxides are made now?
KM: If we look at routes through which epoxides are made, we can look for instance at something like ethylene oxide, the largest volume epoxide that’s made today. For every six ethylenes that become ethylene oxide, one ethylene goes to CO2. It gets over-oxidized during this reaction process. There’s this enormous CO2 footprint built into its production. There are other epoxides that we make through routes that involve, for instance, very hazardous reagents. For instance, things like perbenzoic acid or butyl hydroperoxide. The dangerous part of these reagents is that they have an oxygen-oxygen bond within them and that makes some very unstable and difficult to handle. There been many peroxide-based explosions that have occurred and that’s another reason for which we want to get rid of those sorts of epoxidation reagents. On top of that, some of these routes also create stoichiometric waste products. You’ll introduce a reagent, which adds this oxygen atom on to make the epoxide, but then you end up creating a side waste product that you have to dispose of. And those are all things that we wanted to try to alleviate in the new process that we were creating.
KL: Can you define stoichiometric for me?
KM: Stoichiometric refers to the fact that for every single product you create, you also create an equivalent amount of waste products. Every one product has one molecule of waste product generated with it.
KL: Can you tell me about your solution?
KM: What we dreamt of at the very early stages is that if all that’s really happening in this epoxidation reaction is that we need to place one oxygen atom onto an olefin substrate, then we could get that oxygen from a more sustainable source, from water. Our goal coming into this whole project was to figure out a way to take a double bond, to take water, rip that oxygen atom off of water, and place that oxygen atom on to that double bond. It may seem counterintuitive that you could take something like a double bond—an alkene—and water, mix them together, and get some sort of reaction to occur. But we found a way in which you can use electricity to drive that process. You can apply an electric field to the reaction and use that electric field to drive a reaction that otherwise, from a chemist’s intuition, should not seem to be possible.
KL: Is that electricity where renewable sources like solar and wind come in?
KM: Exactly. It’s just very, very critical for us to find ways of using solar and wind and renewable sources of energy to help drive chemical synthesis. We’ve gotten to the point where solar and wind are much cheaper and I think that’s of great use for decarbonizing a lot of the things that we use in our everyday lives, whether it’s how we get from point A to point B, how we power the lights that we turn on, et cetera. But the more stubborn emissions are going to require very creative ways of using renewable energy to decarbonize their production. That’s where the sort of process that we’re developing comes in, as a means of taking solar and wind energy and using that produce more sustainable molecules and materials.
KL: Could you talk about how you became interested in chemical engineering and more about what led you to this epoxides research to begin with?
KM: I remember at a very young age just being very fascinated by batteries and lightbulbs and circuits and spending a lot of time in my bedroom putting together wires to make little circuits and just seeing how they worked. It was entirely a trial-and-error approach of finding a lightbulb from one product and taking it out and finding a double A battery that was left behind and seeing what made the lightbulb brighter, what made it dimmer, and then making gradually more and more complicated circuits as I got older. That was sort of my first Edisonian love of circuits at a very young age.
As I got older, I also got interested in chemistry. That was much more in high school, I think. That’s when I sort of started to become fascinated with how chemical bonds form and how they break. But I never viewed these interests as being things that I would ultimately bring together, until maybe five or ten years later when I was in graduate school and I found ways in which electricity and chemical bond formation and breaking were actually problems that very much fit together. There was a way in which those interests came together in a way that I never really planned on or expected.
KL: Was there a particular class that brought that together for you?
KM: I think during graduate school it was a realization that there were problems that people had worked on for more than a century, in the area of fuel cells and batteries and electrolyzers. Those of these things I had known since I was a child but as I grew older, it came much more into focus that this was an area in which I myself wanted to contribute to.
KL: Within chemical engineering, what are your specialties?
KM: In chemical engineering, my own focus would often be categorized as catalysis. In this space what we’re interested in is figuring out how to facilitate the transformation of one molecule into another. How to make that occur more quickly. That’s the focus of catalytic research in general. The twist that we bring to it is electric catalysis, using electrons to facilitate this catalytic process through which you accelerate the conversion of one molecule into another.
The big picture that I think gets me excited in the morning when I wake up is just the very simple notion that if all you have is air, water, and renewable electricity, how much can you make of everything that you deal with in your everyday life. I think simplifying almost all the research that we do to create this very sort of aggressive and stringent restriction on what we do has forced us to innovate in ways that we wouldn’t have otherwise. From air you can get carbon from the CO2, you can get nitrogen from the dinitrogen gas, from water you get both hydrogens and oxygens. Now with those carbons, nitrogens, oxygens, and hydrogens, you have atoms that you can now start to stitch together to make more complex things. Now this paradigm may seem overly restrictive. In some ways it is. Because there many other things that you want to do outside of just using air, water, and renewable electricity to make things that you’re interested in. It turns out that many of the advances that we make it through this paradigm end up being useful outside of that paradigm.
The epoxidation of double bonds is one good example of that. How we’re able to take something that emanated from this paradigm to do something that’s outside that paradigm alone. That’s, I think, often the case in the history of chemical synthesis. There are paradigms like total synthesis, for instance. Where the way that paradigm is stated, is that you have to start with commercially available precursors to make any molecule that you’re interested in. That’s often drawn in contrast to using some sort of natural product from nature. Going to the foothills of the Himalayas, finding some extract, and using that as the source of a molecule that you eventually want to make. Now, total synthesis may seem very restrictive, in that you have to start with the commercially available precursor. But it turns out that the advances made there have been useful in other functionalization reactions as well. The same is true for what we’re proposing as well. You don’t have to start with just air, water, and renewable electricity, but you can do much more in terms of other functionalization reactions.
KL: That’s amazing. Just that you all stumbled on this. Not really stumbled but I’m sure went through lot of trial-and-error and failure. Do you have any anecdotes from the process that got you here? Maybe encouraging other scientists who are looking into similar situations?
KM: Absolutely. I think that, for me, is the most difficult part of the journey through graduate school. It’s what you hope that someone takes away after going through that journey, is that for the first time, many students fail for months, if not years, in trying to do something. That can be a very jarring and difficult experience. Especially for someone who’s been used to success in any of the other things that they’ve done before. Starting to understand that through those perceived failures, that that is actually by itself knowledge, in terms of what doesn’t work. To cast that in a positive light, and to also realize that it’s difficult, specifically because it hasn’t been done before. Those are all things that are part of this emotional journey through graduate school that one has to start to stitch together, to endure and get to something that is genuinely new at the frontiers of science and engineering. That, I think, is by far the most difficult thing. I think we often think that it’s the putting together the technical pieces that’s most challenging, but it’s continuing to do what you’re doing every day and to see it through all the technical lessons that you’ve learned in your whole life and to not give up on those at a time of great stress perhaps. To maintain balance in one’s life while doing those things that I think ends up being the most important lesson of the time that one spends at grad school. But certainly, went through many, many failures and many catalysts that don’t work. You can ask most of our students about how many of these dreams that they initially sketch out on a piece of paper or on a whiteboard, many of them don’t come true. But the few that do are just so profoundly impactful and energizing. We learn to celebrate those moments of success but also those moments in which things don’t work exactly lately the way that we want to. By seeing the good in both of those outcomes, I think we create a culture in which we’re able to continue to pursue these very difficult goals that we set forth.
The reactions that we’re trying to replace are ones that are very, very old, that have been perfected over a century, if not longer in many cases. It’s very hard to find replacements to those incumbent technologies that are so entrenched. That’s what we remind ourselves often, is that if we’re disappointed by feeling over of a period of a couple of months, we just have to remember that whatever has already been created has had a hundred years, in many cases, to have been put forth and perfected and to be found to do what it does. We have to be patient often in our approach if we do want to change the world in the way that we want to.
In the early stages, what we’ll start with first is going up to a whiteboard and just starting to lay out this very crazy vision. I think at times we sketch out things that we know in the back of our minds that we won’t hit until 10, 15, or 20 years from now. That’s many generations of graduate students and will certainly take me through a very large part of my career. We then have to step back and start to break that down into things that we can try today, that we want to try a week from now, a few months from now, and maybe a few years from now. That’s the more immediate time scale in which we think, the time scale of a graduate student’s existence within the lab. We then take those and we start running those initial experiments, running catalysts that are based off of design principles that we envision in our minds, and we see what happens. That leads us to them revise that initial picture, that cartoon that we drew, and we can continue to revise that cartoon until it becomes sufficiently detailed that it’s actually predictive. At that point, that’s usually where we start to find success in what we’re doing, because that cartoon is no longer just a dream. It’s something that’s informed by observables that we’ve collected in the lab, and that’s a very powerful moment. But that can sometimes take a few weeks, and more often it takes a few months, if not a few years, to get to the point where a sketch is predictive in power. That’s the difficult part of the journey.
This example of making epoxides is something which we started just by sketching a cartoon of, in terms of how water would come in and how that would liberate an oxygen atom and how that oxygen atom could then do a very specific epoxidation reaction and we had to dream of certain catalysts that could do this. We thought about what other catalysts do we know that are able to rip water apart and that’s something that we then tested and we saw that it had some reasonable yield for epoxidation and then had to gradually work on that catalyst to increase those yields and make it a more effective catalyst for oxidation. If we had looked at just the very first result, we may have very well given up on this problem entirely and gone on to something else, some lower hanging fruits, some more incremental improvement that’s already been done. But because the postdoc working on this project and the graduate student and myself felt deeply that this cartoon that we had sketched should work, and we took the initial observables and kept refining that cartoon, we got to a more predictive version of that hypothesis that actually panned out.
KL: Absolutely. Can you tell us more about how your research specifically led you to epoxides?
KM: We eventually headed towards this system in which a manganese oxide catalyst is able to drive this step of breaking the oxygen and hydrogen bonds in water, taking that liberated oxygen atom, and then transferring it to this double bond to create this triangle in which you have two carbons and an oxygen. That’s the final product that we’re interested in. That catalyst is one which is surprisingly effective at driving what we refer to as “oxygen atom transfer”, as opposed to just letting these oxygen atoms recombine with each other. You can imagine that when you break these oxygen-hydrogen bonds, you can then form oxygen-oxygen bonds, and that in turn would lead to forming O2 as a final product. That would be an undesirable product in this case. Because our goal is to instead use those oxygen atoms to use an epoxide. That’s for instance one design principle that we’ve had to think a bit about, is how to do you prevent O2 from forming selectively.
That actually leads to one more way of looking at this overall system, is that one can think, for instance, about a water electrolyzer, a system which takes water, splits it, making hydrogen and oxygen. We view the hydrogen as a very valuable product, and something that we can sell and use to power a lot of the processes that we use in our everyday lives. But we view the oxygen just as something that we vent to the universe that has no value in it. In a way, we’ve come with a method by which you can bring value to those oxygen atoms, rather than just letting them come together and go off into the universe, we take each oxygen atom and we transfer it to a double bond in order to make an epoxide. That then brings value to those oxygen atoms. In a way, what we’ve created here is a way of making cheaper hydrogen as well, since we’ve now found something to do with those oxygen atoms that otherwise would have just been these useless things that we let go of.
The safety problem I think is important here. Because all we’re bringing in is whatever we want to epoxidize. This double bond, this alkane, olefin, it has many different names which I think sometimes can add to the nomenclature barrier to understand what we’re doing here. But you have these molecules that you’re bringing in that you want to transfer an oxygen atom to, and that oxygen atom just comes from water. There couldn’t be a safer source of those oxygen atoms in water alone. When you compare that to perbenzoic acid or tert-butyl hydroperoxide, much more complicated reagents that have these dangerous oxygen-oxygen bonds within them, we’re able to replace all those reagents with just water alone. That significantly improves the safety of the process.
KL: Most efforts to reduce energy consumption and carbon emissions focus on the transportation and residential sectors but little attention has been paid to the industrial manufacturing sector, which actually consumes more energy than either of the other two sectors. Why do you think that is?
KM: I think things that are directly consumer-facing are sometimes easier to tackle first. It’s in the conscience of the consumer, right? That they have a choice of whether they buy a Honda Accord or a Tesla Model 3. These are choices that they can see right in front of them and that brings for them into focus that there is this trend of electrification in transportation. Or that they have a choice with their utility. A choice that the utility presents to them, perhaps. Whether they continue to buy the usual grid mix or want their electricity to come a solar panel. These are things that are in front of the consumer. We tend to think a lot less when we’re looking through our closet in the morning and deciding what to put on, as to whether this blue dress shirt has a lower CO2 footprint than the white one. We haven’t been educated to think about the choices, about the CO2 footprint that’s embedded in that choice that we make there. Or when we’re at Nordstrom deciding what we want to purchase. That there is a CO2 footprint associated with that. That’s important. Or in the food that we purchase at the store. That the fertilizer is used to produce a given vegetable can have an enormous CO2 footprint in one case, or if it’s using newer technology, could entirely remove that CO2 footprint involved just in the fertilization of that crap. All of these emissions that we’re not yet educated in terms of how to make choices between, or in some cases we just don’t have the technologies yet to remove the CO2 emissions. In most cases we don’t have the technologies to remove those CO2 footprints yet. But gradually, I think, it’ll come into the mindset of the consumer that these choices are in front of them. In some cases, consumers will be willing to pay a premium as an early adopter to see something happen.
But what we really hope eventually is that these are things that are so widespread and that become so cheap that the consumer doesn’t even have to think about it. That when they’re drinking a bottle of water, they don’t have to worry as to whether that’s polyethylene terephthalate that came from a conventional process, which has a very large CO2 emission associated with it, or whether it comes from a new green process that’s somehow CO2 negative. To the extent to which this become seamless and isn’t behavior-dependent on the consumer, that’s eventually the world that we want to see. But that will be a very gradual transition.
KL: I just have to say I’m really surprised, given the consumer focus of the transportation sector and the residential sector that it hasn’t come in to our conversations about our clothes and our bottled water and the laundry detergent that we’re washing our clothes in. It’s just fascinating to me. Why does it hit certain aspects of our lives first?
KM: I think we’re at a turning point where people are just starting to think about these things. They see some choices in front of them. I think the next few years we’ll see a big change in terms of that.
KL: I hope so. What’s next for you and your team?
KM: We’re continuing to push this paradigm forward, in terms of what can we do with just air, water, and renewable electricity. We’re, for instance, finding ways to make ammonia in this matter. Where you take N2 from the air, you take hydrogens from water, and then you can make ammonia as a fertilizer. This is a much greener and cleaner way of making fertilizers than has historically been practiced. At present, the ammonia that we synthesize as a fertilizer has a very large CO2 footprint. In fact, the number one CO2 footprint of any chemical that we make today. That ends up being a very important problem to solve. There are other examples along with that, in terms of making other monomers for plastics, pharmaceuticals, all of which we want to be decarbonized, but also made safer, more modular, on-demand methods that can create this electrification and decarbonization of the chemical industry, ultimately.
KL: You mentioned that this was a counterintuitive approach, earlier. Why hasn’t this approach been done before?
KM: I think the first aspect that I want to mention is that we can treat many of the new processes that we’re creating. We can categorize them as electric chemical processes. These are processes in which electricity is used to affect a chemical reaction. Many of the reactions that we’re competing against are thermochemical reactions. These are ones in which temperature and pressure are generally used to affect a chemical reaction or to cause it to occur.
The major advantage of thermochemical reactions is that you put these reagents in a vessel and you apply temperature and pressure and the reaction occurs. Those reactions scale volumetrically, whereas the reactions that we’re creating scale with area. Because they involve an electrode and that electrode scales as r2 as you increase its size or decrease its size. The volumetric reactor scale is r3, and so as you increase radius or you increase this scale, you can get to much larger scales of production much more easily with a volumetric thermochemical process, as opposed to an aerial electro-chemical process. That scaling is at the very heart of why if there is a thermochemical way of doing something, one has generally historically preferred to do that thermochemical reaction at scale in a centralized fashion.
Now, the paradigm that we’re putting forth is that you don’t need to do things at massive scale. If you take whatever advantage you get from volumetric scaling and doing it in a decentralized fashion and then having distribute it, and you instead say, if we make it locally at a smaller scale but we no longer need to distribute it and we just have it made there on-demand with no need for inventory, that there are certain advantages to be gained in doing that. Even with the aerial scaling, the fact that you can make it on-demand and not have to distribute it can create a new advantage for these sorts of processes. It depends entirely on the reaction that you’re doing and the product that you’re making as to whether that volumetric scaling versus aerial scaling ends up being an advantage or a disadvantage. But that’s a very important consideration. Not all reactions will become electrochemical. Some subset of the chemicals that we make today will become electrochemical and others will rightfully so remain thermochemical processes because that is the right way to do those particular reactions.
I think on the epoxides research, the thing which we really want to stress is that if you look at how water electrolysis research was done for a long time, it was done within a very fixed confine of generating hydrogen on one side and generating oxygen on the other. Even though one was just so used to seeing that overall reaction written, as H2O goes to H2 plus O2, that even that was something that could be revised in terms of an overall reaction to create a new process by which you could still create the hydrogen but create something else useful too. Sometimes the more comfortable we get with the paradigm, the more ripe it is for being disrupted or changed. I think that is something that to us is comforting and discomforting at the same time.
KL: If your solution becomes mainstream, many years down the road, would there be results, either large or small scale, that the average person would experience?
KM: I think in some ways if we’re successful to the extent that we want to be and we create a method of making chemicals and materials that doesn’t have a carbon footprint, that’s safer to produce, that can be made on-demand, that these are all things that would hopefully create replacements which the consumer wouldn’t even realize that we have swapped something out. That this plastic bottle that we’re drinking water out now no longer has a CO2 footprint without the consumer even needing to know that that happened because this new replacement is cheaper and better than what existed before. We know in all reality that the way that cost curves work and in the way that new technologies get implemented, that that would be a very rare occurrence and so there will be things that consumers see put in front of them, marketing-wise, as consumers grow more conscious of the benefits of more sustainable chemicals in materials.
KL: We’ll link to your paper in the show notes for this episode. Where else can people go to learn more about your research or about this topic?
KM: Our lab’s website, manthiram.mit.edu, is a good place to start to learn about this. We’re also on Twitter @manthiramlab. That’s another place where you’ll see us posting new things that we’ve found and discovered but also finding ways to help bring the community together in this space. We also published an article recently on the electrification and decarbonization of the chemical industry. That’s another place that I think is a good starting point for understanding some of the trends that we may see in the next five to ten years. Both in terms of how we deliver clean electricity to the consumer but also in terms of how we decarbonize the chemicals and materials that we use every day.
KL: Great. Thank you so much for being here today, Karthish.
KM: Awesome, thank you.
In brief
Making raw materials for the manufacture of consumer goods produces high levels of carbon dioxide (CO2) emissions, involves hazardous materials, and requires high temperatures and pressures, usually generated by burning fossil fuels. MIT chemical engineers have now demonstrated a new approach that can operate on water plus electricity from renewable sources. Energized by a well-known catalyst, the process forms no CO2 emissions, requires no hazardous materials or extreme operating conditions, and generates just one byproduct—hydrogen. While much work remains, this new approach—relying on electricity and electrocatalysts—could one day significantly reduce the vast amounts of CO2 produced by the chemical industry today.
Most efforts to reduce energy consumption and carbon emissions have focused on the transportation and residential sectors. Little attention has been paid to industrial manufacturing, even though it consumes more energy than either of those sectors and emits high levels of CO2 in the process.

To help address that situation, Assistant Professor Karthish Manthiram, postdoc Kyoungsuk Jin, graduate students Joseph H. Maalouf and Minju Chung, and their colleagues, all of chemical engineering, have been devising new methods of synthesizing epoxides, a group of chemicals used in the manufacture of consumer goods ranging from polyester clothing, detergents, and antifreeze to pharmaceuticals and plastics.
“We don’t think about the embedded energy and carbon dioxide footprint of a plastic bottle we’re using or the clothing we’re putting on,” says Manthiram. “But epoxides are everywhere!”
As solar and wind and storage technologies mature, it’s time to address what Manthiram calls the “hidden energy and carbon footprints of materials made from epoxides.” And the key, he argues, may be to perform epoxide synthesis using electricity from renewable sources along with specially designed catalysts and an unlikely starting material: water.
The challenge
Epoxides can be made from a variety of carbon-containing compounds known generically as olefins. But regardless of the olefin used, the conversion process generally produces high levels of CO2 or has other serious drawbacks.
To illustrate the problem, Manthiram describes processes now used to manufacture ethylene oxide, an epoxide used in making detergents, thickeners, solvents, plastics, and other consumer goods. Demand for ethylene oxide is so high that it has the fifth-largest CO2 footprint of any chemical made today.
The top panel in the figure below shows one common synthesis process. The recipe is simple: Combine ethylene molecules and oxygen molecules, subject the mixture to high temperatures and pressures, and separate out the ethylene oxide that forms.

However, as the diagram shows, those ethylene oxide molecules are accompanied by molecules of CO2—a problem, given the volume of ethylene oxide produced nationwide. In addition, the high temperatures and pressures required are generally produced by burning fossil fuels. And the conditions are so extreme that the reaction must take place in a massive pressure vessel. The capital investment required is high, so epoxides are generally produced in a central location and then transported long distances to the point of consumption.
Another widely synthesized epoxide is propylene oxide, which is used in making a variety of products, including perfumes, plasticizers, detergents, and polyurethanes. In this case, the olefin—propylene—is combined with tert-butyl hydroperoxide, as illustrated in the bottom panel above. An oxygen atom moves from the tert-butyl hydroperoxide molecule to the propylene to form the desired propylene oxide. The reaction conditions are somewhat less harsh than in ethylene oxide synthesis, but a side product must be dealt with. And while no CO2 is created, the tert-butyl hydroperoxide is highly reactive, flammable, and toxic, so it must be handled with extreme care.
In short, current methods of epoxide synthesis produce CO2, involve dangerous chemicals, require huge pressure vessels, or call for fossil fuel combustion. Manthiram and his team believed there must be a better way.
A new approach
The goal in epoxide synthesis is straightforward: Simply transfer an oxygen atom from a source molecule onto an olefin molecule. Manthiram and his lab came up with an idea: Could water be used as a sustainable and benign source of the needed oxygen atoms? The concept was counterintuitive. “Organic chemists would say that it shouldn’t be possible because water and olefins don’t react with one another,” he says. “But what if we use electricity to liberate the oxygen atoms in water? Electrochemistry causes interesting things to happen—and it’s at the heart of what our group does.”
Using electricity to split water into oxygen and hydrogen is a standard practice called electrolysis. Usually, the goal of water electrolysis is to produce hydrogen gas for certain industrial applications or for use as a fuel. The oxygen is simply vented to the atmosphere.
To Manthiram, that practice seemed wasteful. Why not do something useful with the oxygen? Making an epoxide seemed the perfect opportunity—and the benefits could be significant. Generating two valuable products instead of one would bring down the high cost of water electrolysis. Indeed, it might become a cheaper, carbon-free alternative to today’s usual practice of producing hydrogen from natural gas. The electricity needed for the process could be generated from renewable sources such as solar and wind. There wouldn’t be any hazardous reactants or undesirable byproducts involved. And there would be no need for massive, costly, and accident-prone pressure vessels. As a result, epoxides could be made at small-scale, modular facilities close to the place they’re going to be used—no need to transport, distribute, or store the chemicals produced.
Will the reaction work?
However, there was a chance that the proposed process might not work. During electrolysis, the oxygen atoms quickly pair up to form oxygen gas. The proposed process—illustrated in the diagram below—would require that some of the oxygen atoms move onto the olefin before they combine with one another.

To investigate the feasibility of the process, Manthiram’s group performed a fundamental analysis to find out whether the reaction is thermodynamically favorable. Does the energy of the overall system shift to a lower state by making the move? In other words, is the product more stable than the reactants were?
They started with a thermodynamic analysis of the proposed reaction at various combinations of temperature and pressure—the standard variables used in hydrocarbon processing. As an example, they again used ethylene oxide. The results, shown below, were not encouraging. As the uniform blue in the left-hand figure shows, even at elevated temperatures and pressures, the conversion of ethylene and water to ethylene oxide plus hydrogen doesn’t happen—just as a chemist’s intuition would predict.
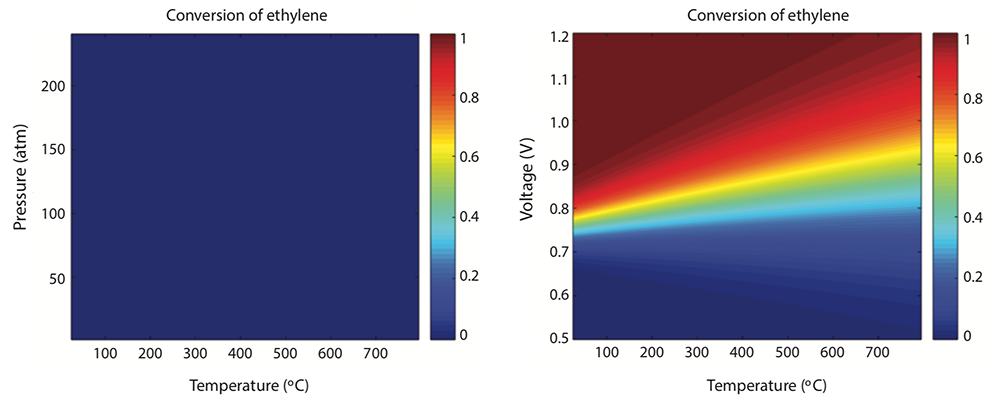
But their proposal was to use voltage rather than pressure to drive the chemical reaction. As the right-hand figure above shows, with that change, the outcome of the analysis looked more promising. Conversion of ethylene to ethylene oxide occurs at around 0.8 volts. So the process is viable at voltages below that of an everyday AA battery and at essentially room temperature.
While a thermodynamic analysis can show that a reaction is possible, it doesn’t reveal how quickly it will occur, and reactions must be fast to be cost-effective. So the researchers needed to design a catalyst—a material that would speed up the reaction without getting consumed.
Designing catalysts for specific electrochemical reactions is a focus of Manthiram’s group. For this reaction, they decided to start with manganese oxide, a material known to catalyze the water-splitting reaction. And to increase the catalyst’s effectiveness, they fabricated it into nanoparticles—a particle size that would maximize the surface area on which reactions can take place.
The diagram below shows the special electrochemical cell they designed. Like all such cells, it has two electrodes—in this case, an anode where oxygen is transferred to make an olefin into an epoxide, and a cathode where hydrogen gas forms. The anode is made of carbon paper decorated with the nanoparticles of manganese oxide (shown in yellow). The cathode is made of platinum. Between the anode and the cathode is an electrolyte that ferries electrically charged ions between them. In this case, the electrolyte is a mixture of a solvent, water (the oxygen source), and the olefin.

The magnified views show what happens at the two electrodes. The right-hand view shows the olefin and water (H2O) molecules arriving at the anode surface. Encouraged by the catalyst, the water molecules break apart, sending two electrons (negatively charged particles, e–) into the anode and releasing two protons (positively charged hydrogen ions, H+) into the electrolyte. The leftover oxygen atom (O) joins the olefin molecule on the surface of the electrode, forming the desired epoxide molecule.
The two liberated electrons travel through the anode and around the external circuit (shown in red), where they pass through a power source—ideally, fueled by a renewable source such as wind or solar—and gain extra energy. When the two energized electrons reach the cathode, they join the two protons arriving in the electrolyte and—as shown in the left-hand magnified view—they form hydrogen gas (H2), which exits the top of the cell.
Experimental results
Experiments with that setup have been encouraging. Thus far, the work has involved an olefin called cyclooctene, a well-known molecule that’s been widely used by people studying oxidation reactions. “Ethylene and the like are structurally more important and need to be solved, but we’re developing a foundation on a well-known molecule just to get us started,” says Manthiram.
Results have already allayed a major concern. In one test, the researchers applied 3.8 volts across their mixture at room temperature, and after 4 hours, about half of the cyclooctene had converted into its epoxide counterpart, cyclooctene oxide. “So that result confirms that we can split water to make hydrogen and oxygen and then intercept the oxygen atoms so they move onto the olefin and convert it into an epoxide,” says Manthiram.
But how efficiently does the conversion happen? If this reaction is perfectly efficient, one oxygen atom will move onto an olefin for every two electrons that go into the anode. Thus, one epoxide molecule will form for each hydrogen molecule that forms. Using special equipment, the researchers counted the number of epoxide molecules formed for each pair of electrons passing through the external circuit to form hydrogen.
That analysis showed that their conversion efficiency was 30% of the maximum theoretical efficiency. “That’s because the electrons are also doing other reactions—maybe making oxygen, for instance, or oxidizing some of the solvent,” says Manthiram. “But for us, 30% is a remarkable number for a new reaction that was previously unknown. For that to be the first step, we’re very happy about it.”
Manthiram recognizes that the efficiency might need to be twice as high or even higher for the process to be commercially viable. “Techno-economics will ultimately guide where that number needs to be,” he says. “But I would say that the heart of our discoveries so far is the realization that there is a catalyst that can make this happen. That’s what has opened up everything that we’ve explored since the initial discovery.”
Encouraging results and future challenges
Manthiram is cautious not to overstate the potential implications of the work. “We know what the outcome is,” he says. “We put olefin in, and we get epoxide out.” But to optimize the conversion efficiency they need to know at a molecular level all the steps involved in that conversion. For example, does the electron transfer first by itself, or does it move with a proton at the same time? How does the catalyst bind the oxygen atom? And how does the oxygen atom transfer to the olefin on the surface of the catalyst?

According to Manthiram, he and his group have hypothesized a reaction sequence, and several analytical techniques have provided a “handful of observables” that support it. But he admits that there is much more theoretical and experimental work to do to develop and validate a detailed mechanism that they can use to guide the optimization process. And then there are practical considerations, such as how to extract the epoxides from the electrochemical cell and how to scale up production.
Manthiram believes that this work on epoxides is just “the tip of the iceberg” for his group. There are many other chemicals they might be able to make using voltage and specially designed catalysts. And while some attempts may not work, with each one they’ll learn more about how voltages and electrons and surfaces influence the outcome.
He and his team predict that the face of the chemical industry will change dramatically in the years to come. The need to reduce CO2 emissions and energy use is already pushing research on chemical manufacturing toward using electricity from renewable sources. And that electricity will increasingly be made at distributed sites. “If we have solar panels and wind turbines everywhere, why not do chemical synthesis close to where the power is generated, and make commercial products close to the communities that need them?” says Manthiram. The result will be a distributed, electrified, and decarbonized chemical industry—and a dramatic reduction in both energy use and CO2 emissions.
This research was supported by MIT’s Department of Chemical Engineering and by National Science Foundation Graduate Research Fellowships. Further information can be found in:
K. Jin, J.H. Maalouf, N. Lazouski, N. Corbin, D. Yang, and K. Manthiram. “Epoxidation of cyclooctene using water as the oxygen atom source at manganese oxide electrocatalysts.” Journal of the American Chemical Society, vol. 141, pp. 6413–6418, 2019. Online: doi.org/10.1021/jacs.9b02345.
Z.J. Schiffer and K. Manthiram. “Electrification and decarbonization of the chemical industry.” Joule, vol. 1, pp. 10–14, September 6, 2017. Online: doi.org/10.1016/j.joule.2017.07.008.
This article appears in the Autumn 2019 issue of Energy Futures.